Topics
Our Areas of Interest
Understanding and Developing Nickel Complexes for Catalysis
We aim to understand how active catalysts form in situ and to design new and more effective nickel catalysts.
Catalyst Activation
Nickel catalysis is typically performed by adding a nickel(0) or nickel(II) source and a ligand, and relying on the formation of an active species in situ. We aim to understand the nature and reactivity of all of the species that might form under these scenarios, and understand how we can promote the formation of the most active complexes. This work is supported by studentship collaborations with AstraZeneca and GSK.
New Catalysts
Work to prepare and understand the reactivity of low-valent nickel complexes has led to the discovery of a suite of robust nickel(0) catalysts that can be prepared cheaply, safely, and on scale. We are currently exploring opportunities to bring these catalysts to market, supported by colleagues in Innovation and Industry Engagement at the University of Strathclyde (Dr Emma Millhouse), an experienced commercial champion (Dr Ian Muirhead), and funded by organisations including Innovate UK (via the ICURe programme).
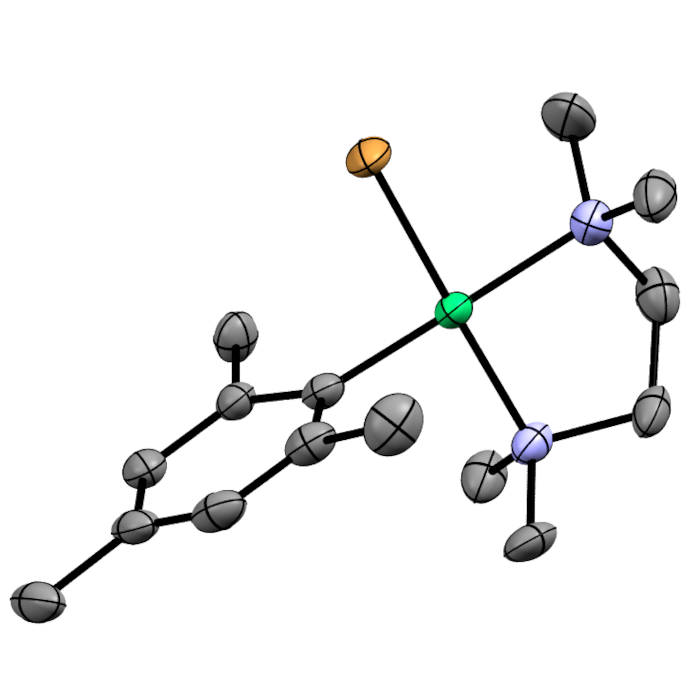
Functional Groups in Nickel Catalysis
We aim to understand how nickel catalysts interact with the functional groups present in the molecules that synthetic chemists may wish to make.
Aldehydes and Ketones
Nickel binds more strongly to aldehydes and ketones than palladium does, which leads to a number of effects in catalysis. Depending on the locus of the aldehyde or ketone within the reaction, this can result in reaction inhibition, or the control of site-selectivity.
Heteroaryl Halides
Pyridyl halides, where the halogen is in the 2-position, can form dimeric nickel(II) complexes upon reaction with nickel(0); these were found to be inactive for Suzuki-Miyaura cross-coupling reactions. However, these will undergo transmetalation with (more reactive) species such as arylmagnesium halide reagents.
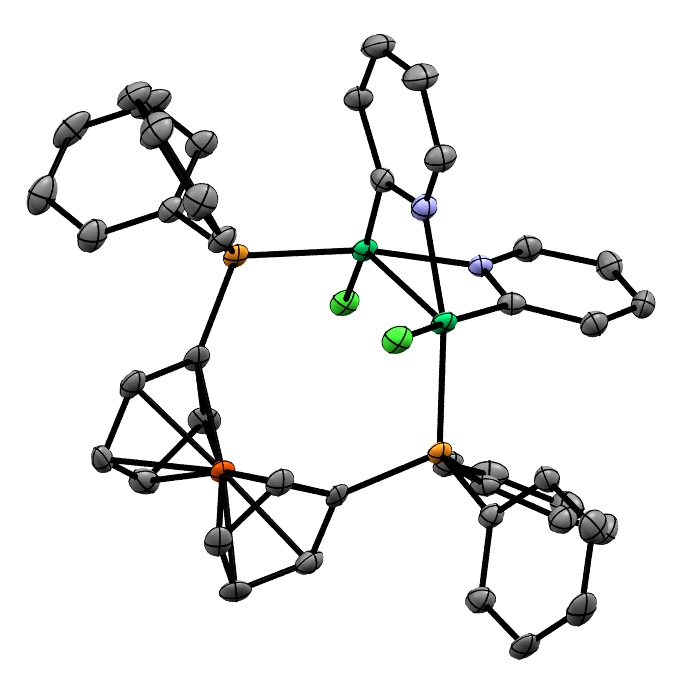
Structure/Reactivity Relationships in Oxidative Addition to Nickel
We aim to understand the mechanisms by which nickel(0) and nickel(I) complexes undergo oxidative addition, and how the rates and mechanisms of these reactions depend on structural features of the nickel complex and the substrate.
Reactions of [Ni(COD)(dppf)] with Aryl, Alkenyl, and Alkyl (Pseudo)Halides
We have studied the reactivity of this complex in significant depth, and have explored its reactions with aryl (psuedo)halides, aryl and alkenyl triflates, and alkyl halides. The active species is not always the same: the reactions with alkyl halides proceed via [Ni(dppf)2].
[Ni(PR3)4] Complexes: Nickel(I) versus Nickel(II) Products
We have used DFT calculations to re-examine the seminal work of Kochi and to understand why some reactions give nickel(I) products and others give nickel(II). The key determinant of selectivity is the strength of the C-X bond in the aryl halide substrate; halide abstraction leads to nickel(I) while oxidative addition gives nickel(II).
Steric Effects in the Reactions of [Ni(NHC)2] with Aryl Halides
We have explored [Ni(NHC)2] systems using computational methods, and have shown that the key factor is the steric size of the NHC ligand. Smaller NHC ligands allow oxidative addition to proceed, but larger NHC ligands preclude oxidative addition and so halide abstraction takes place (to form nickel(I)).
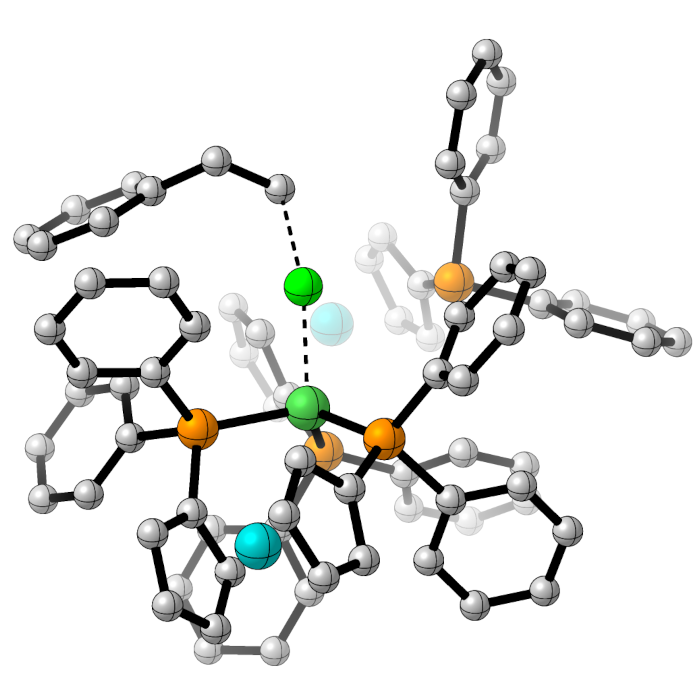
Understanding Selectivity in C-H Activation Reactions
Most substrates for synthetic chemistry have many C-H bonds, often in very similar environments, so ensuring selectivity in C-H activation reactions is an enduring challenge.
Applying Machine Learning to C-H Activation Chemistry
We are currently collaborating with GSK to train and deploy a machine learning model to understand the behaviour of widely-used C-H activation reactions.
Selectivity in Iridium-Catalysed Hydrogen Isotope Exchange Reactions
In collaboration with Professor Billy Kerr and Dr David Lindsay (University of Strathclyde) we have developed 'reactivity scales' for the strength of various Lewis basic directing groups for site-selective hydrogen isotope exchange. These are not correlated to the relative rate of reaction.
Selectivity in Ruthenium-Catalysed Arylation Reactions
Prior to our work on hydrogen isotope exchange, we have applied methodology to quantify site selectivity in directed C-H arylation catalysed by [Ru(O2CMes)2(p-cymene)]. In collaboration with Professors Albert Poater and Pedro Salvador (University of Girona) we showed that the selectivity observed could be explained by loss of the p-cymene ligand in the first turnover during the catalytic cycle.
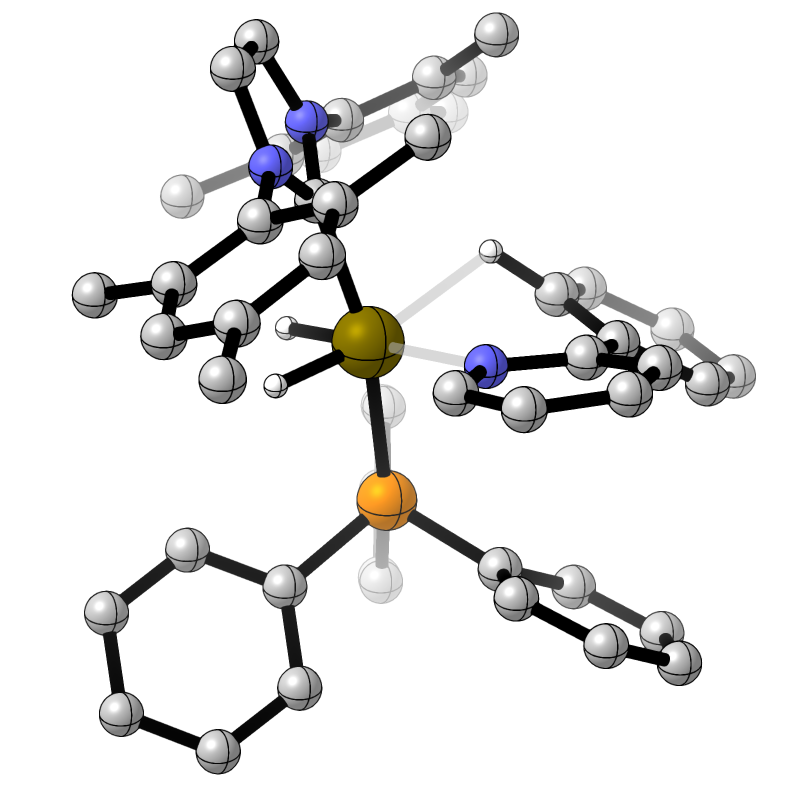
Ligands and Ligand Effects in Organometallic Chemistry and Catalysis
Ligand choice is crucial for most transition metal-catalysed reactions, so we aim to explore the design and application of new ligands and the understanding and quantification of their properties.
N-Heterocyclic Carbenes
N-heterocyclic carbenes are typically straightforward to prepare and can have a vast range of steric and electronic properties. We have an interest in relating NHC structure to the properties of the corresponding metal complexes, and in the design and deployment of new NHC ligands.
Selenoureas Derived from N-Heterocyclic Carbenes
In collaboration with Professors Fady Nahra (VITO), Kristof van Hecke (Ghent), and Steve Nolan (Ghent) we are interested in using selenium derivatives of NHCs to both probe the properties of the corresponding NHCs and as ligands in their own right.
Funding, Collaborations, and Consultancy
We collaborate widely with colleagues in academia and industry, and undertake consultancy work for the chemical industry
We are grateful to the organisations that have supported our work, including:
- Royal Society of Chemistry: Undergraduate Research Bursary [A. Bell] (2024)
- Leverhulme Trust: Project Grant RPG-2024-074 (2024-2027)
- Eli Lilly: Research Award Programme (2024-2026)
- GSK: EPSRC ICASE Studentship (2024-2028)
- AstraZeneca: Collaborative Studentship (2023-2027)
- Syngenta: EPSRC ICASE Studentship (2021-2025)
- GSK: EPSRC ICASE Studentship (2021-2024)
- Royal Society: Research Grant RGS\R1\191483 (2019)
- Carnegie Trust for the Universities of Scotland: Research Incentive Grant RIG008165 (2018)
- Leverhulme Trust: Project Grant RPG-2018-207 (2019-2022)
- GSK: Summer Studentship [F. Bugden] (2018)
- Royal Society of Chemistry: Undergraduate Research Bursary [N. Nabi] (2017)
- EPSRC DTP: Vacation Bursary [M. Cowie] (2017)
- Carnegie Trust for the Universities of Scotland: Research Incentive Grant (2017)
- AstraZeneca: EPSRC ICASE Studentship (2017-2021)
- Syngenta: EPSRC ICASE Studentship (2016)
- Santander: Mobility Fund [I. Mayoral Soler] (2016)
- EPSRC DTP: Vacation Bursary [G. Laidlaw] (2016)
- EPSRC: First Grant EP/M027678/1 (2015-2016)
- Carnegie Trust for the Universities of Scotland: Vacation Scholarship [L. MacDougall] (2015)
- University of Strathclyde: Global Engagement Fund (2015)
- WestCHEM: ECR Travel Funding (2015)
- University of Strathclyde: Chancellor's Fellowship (2014-2018)
